Introduction
In recent years, nitrosamine impurities in pharmaceuticals have emerged as a major global health concern due to regulatory actions. Their presence at even trace levels has led to product recalls, regulatory warnings, and heightened scrutiny by agencies like the FDA, EMA, and Health Canada. At ResolveMass Laboratories Inc., we are expert in the risk assessment, identification, and quantification of nitrosamine impurities in pharmaceuticals, helping Market Authorization Holders meet regulatory expectations and ensure patient safety.
Explore our nitrosamine impurity analysis services ➔
What Are Nitrosamine Impurities in Pharmaceuticals?
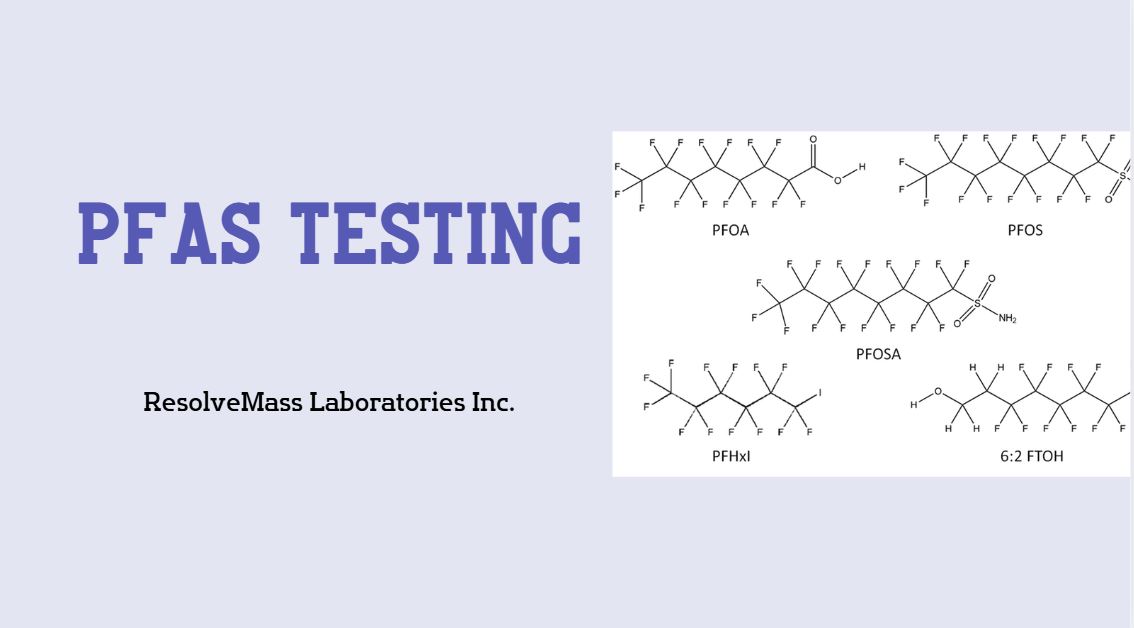
Nitrosamines are chemical compounds typically formed through reactions between amines and nitrosating agents. While some nitrosamines occur naturally in foods and the environment, their appearance as nitrosamine impurities in pharmaceuticals is highly concerning due to their classification as probable human carcinogens.
Commonly detected nitrosamines in drugs include:
- NDMA (N-Nitrosodimethylamine)
- NDEA (N-Nitrosodiethylamine)
- NMBA (N-Nitroso-N-methyl-4-aminobutyric acid)
- NDEA (N-nitrosodiethylamine)
- NEIPA (N-nitrosoethylisopropylamine)
- NDIPA (N-nitrosodiisopropylamine)
- NDBA (N-nitrosodibutylamine)
- NMBA (N-nitroso-N-methyl-4-aminobutanoic acid)
- MNP (1-methyl-4-nitrosopiperazine)
The ability to detect these compounds accurately is crucial, which is why our team uses cutting-edge techniques such as LC-MS/MS and GC-MS to perform sensitive and reliable nitrosamine impurities in pharmaceuticals testing.
Learn about our advanced testing techniques ➔
Why Are Nitrosamine Impurities in Pharmaceuticals a Growing Concern?
1. Carcinogenic Risks
Many nitrosamines are linked to cancer development in humans. Chronic exposure to even low levels significantly raises cancer risk, making rigorous testing essential.
2. Global Recalls
Several widely-used medications, such as certain ARBs (angiotensin II receptor blockers) like valsartan, have faced massive recalls due to nitrosamine impurities in pharmaceuticals.
3. Stricter Regulatory Requirements
Authorities worldwide have set extremely low acceptable intake limits (as low as 18 ng/day for NDMA). Pharmaceutical companies must now prove that their products meet these tough standards.
Discover how we help ensure regulatory compliance ➔
4. Complex Formation Mechanisms
Nitrosamines can form under various conditions, such as during drug synthesis, formulation, or even storage. Understanding and controlling these risks demands specialized expertise.
Sources of Nitrosamine Impurities in Pharmaceuticals
- API Synthesis: Certain solvents, reagents, and raw materials used in Active Pharmaceutical Ingredient (API) production can introduce nitrosamines.
- Formulation Changes: pH adjustments and excipient interactions might trigger nitrosamine formation.
- Packaging Materials: Nitrosating agents in packaging can react with drug substances.
- Degradation Products: Over time, APIs may degrade into nitrosamine compounds under specific storage conditions.
Learn about our full impurity profiling services ➔
Regulatory Guidelines for Nitrosamine Impurities in Pharmaceuticals
Regulatory bodies such as the U.S. FDA, EMA, and ICH have issued strict guidance for identifying, controlling, and mitigating nitrosamine impurities in pharmaceuticals:
- Conduct risk assessments for all products
- Confirm findings with sensitive validated testing methods
- Implement mitigation strategies, including process redesign if necessary
- Establish strict control strategies for manufacturing
Access compliant testing strategies ➔
How ResolveMass Laboratories Inc. Helps
At ResolveMass Laboratories Inc., we offer a comprehensive solution for detecting and managing nitrosamine impurities in pharmaceuticals, including:
- Risk assessment and gap analysis
- Development and validation of sensitive analytical methods
- Routine testing and lot release testing
- Regulatory support and consultation
Partner with the experts in nitrosamine analysis ➔
Real-World Case Study: Successful Mitigation of Nitrosamine Impurities
Challenge:
A leading pharmaceutical company discovered potential nitrosamine impurities in pharmaceuticals during routine quality checks for a common antihypertensive drug.
Solution:
- Rapid implementation of GC-MS/MS testing at ResolveMass Laboratories
- Identified trace amounts of NDMA and NDEA (< 2 ppm)
- Redesigned synthetic pathway with alternate reagents
- Developed validated method with LOQ (Limit of Quantification) of 0.2 ppm
- Full regulatory submission and approval within 6 months
Outcome:
Avoided product recall and improved brand credibility globally.
Learn how we solve real-world challenges ➔
FAQs on Nitrosamine Impurities in Pharmaceuticals
1. What are nitrosamine impurities in pharmaceuticals?
Nitrosamine impurities are carcinogenic chemical contaminants that may form during the manufacturing or storage of pharmaceutical products.
2. Why are nitrosamine impurities dangerous?
Many nitrosamines are classified as probable human carcinogens, meaning they can significantly increase cancer risk with prolonged exposure.
3. How do nitrosamine impurities form?
They typically form through chemical reactions between secondary or tertiary amines and nitrosating agents during synthesis, formulation, or storage.
4. Which drugs are at high risk of nitrosamine contamination?
Medications like ARBs, ranitidine, and metformin have historically been vulnerable due to their synthesis routes and storage conditions. Also, drugs with structures with amines and having nitrosating agents in the synthesis process are at highest risk.
5. What are regulatory acceptable limits for nitrosamines?
Limits are extremely low, with the FDA recommending daily intake limits like 96 ng for NDMA and 26.5 ng for NDEA. The details of Acceptable intakes can be found on various regulatory authorities website like FDA, Health Canada and EMA.
6. How are nitrosamine impurities detected?
Highly sensitive techniques such as LC-MS/MS and GC-MS/MS are used for the accurate detection and quantification of nitrosamine impurities.
7. Can nitrosamine levels increase over time?
Yes, improper storage conditions or chemical degradation can lead to higher nitrosamine levels over time.
8. How can manufacturers mitigate nitrosamine risks?
Mitigation strategies include alternative synthesis pathways, use of high-purity reagents, tighter process controls, and robust packaging systems.
9. What happens if nitrosamine impurities are detected?
Immediate risk assessment, notification to health authorities, potential recalls, and revalidation of manufacturing processes are required.
10. Why choose ResolveMass Laboratories Inc. for nitrosamine testing?
We offer unmatched expertise, validated methods, rapid turnaround times, and regulatory support for effective management of nitrosamine impurities in pharmaceuticals.
Contact our scientific experts today ➔
Conclusion
The growing concern around nitrosamine impurities in pharmaceuticals is justified due to their potent carcinogenic potential and the evolving regulatory landscape. Early detection, expert analysis, and strategic mitigation are the pillars of ensuring product safety and protecting public health. By partnering with a trusted laboratory like ResolveMass Laboratories Inc., pharmaceutical companies can confidently navigate these challenges and secure their market positions.
Get in touch with our expert team to proactively manage your risk.
Contact ResolveMass Laboratories Inc. ➔
ResolveMass Laboratories Inc.: Experience, Expertise, and Trust You Can Count On
ResolveMass Laboratories Inc. is a leading name in nitrosamine testing across the United States and Canada. With over a decade of experience, our PhD-level scientists specialize in Mass Spectrometry and nitrosamine impurity chemistry. We offer complete in-house solutions, including:
- Risk assessment
- Confirmatory analysis
- Regulatory documentation
- Expert consultation
We also offer AI-enhanced nitrosamine testing workflows for faster, more efficient analysis. As one of the few Canadian CROs, we also provide custom synthesis of rare nitrosamine impurities unavailable elsewhere.
Choose ResolveMass Laboratories for precise and transparent nitrosamine testing services.
Ready to Get Started?
📩 Contact our expert team
📞 Request a quote for method development
📅 Book a consultation with our scientists
🧪 Submit your sample for testing
References
- EMA. (2021). Assessment and Mitigation of Nitrosamine Risk in Human Medicines. https://www.ema.europa.eu/en/documents/referral/nitrosamines-emea-h-a53-1490-assessment-report_en.pdf
- FDA. (2021). Control of Nitrosamine Impurities in Human Drugs. https://www.fda.gov/media/141720/download
Polymer Analysis Services & Laboratories
Introduction When clients search for a Polymer Analysis Laboratory, they want accurate, reliable, and timely…
Polymer Deformulation and Contamination Analysis
Introduction Polymer Deformulation Characterization is the scientific method of breaking down and studying polymers to…
Analytical Characterization of Polymers (Spectroscopy & Scattering)
Introduction The accurate study of polymers through analytical methods is crucial for understanding their molecular…
Polymer Thermal Analysis and Property Measurement
Introduction Polymer Thermal Analysis is essential for understanding how different polymers respond to changes in…
Advanced Polymer Molecular Weight Characterization
Introduction HMW Polymer Characterization plays a central role in modern material science, since polymer molecular…
Polymer Composition and Structure Analysis
Introduction Polymer Composition Analysis is the foundation for understanding how polymers behave in different industries,…